TECHNOLOGY
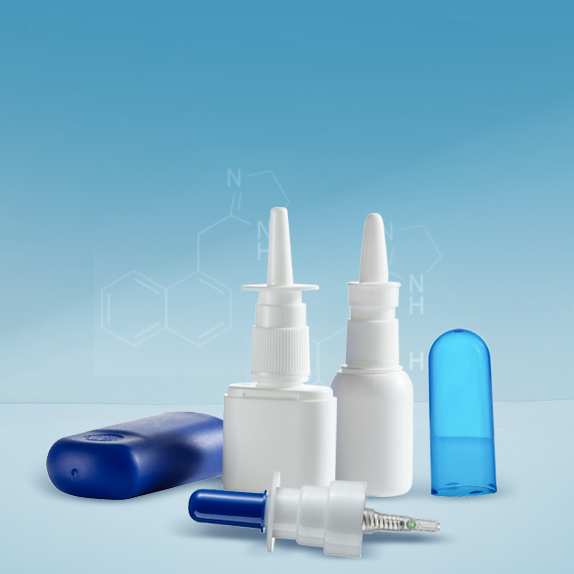
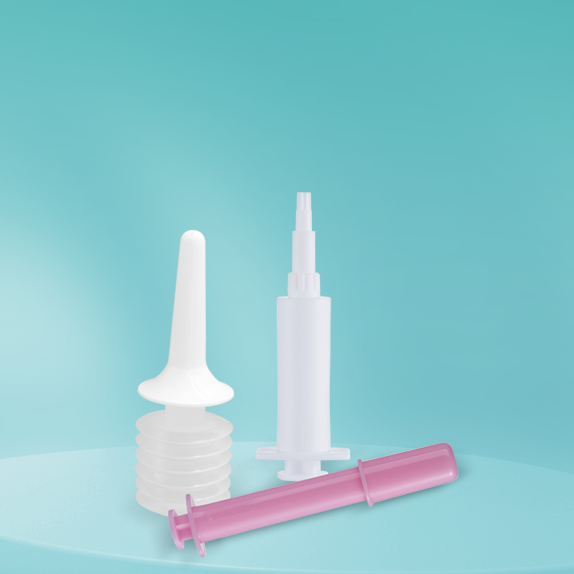
For more than 18 years, Stone has shown the professional R&D capability and manufacturing strength in the drug delivery and pharmaceutical packaging materials. We have our own mold and molding technology, intelligent production and inspection equipment, physical, chemical and microbiological experimental equipment and related talents, and with strong product developed capabilities, we can quickly and efficiently meet various needs of customers.
More+